Our current work focuses on two forms of diabetes:
1. Type 1 diabetes
Our work in type 1 diabetes is aimed at developing biomarkers for the natural history of type 1 diabetes and for response to immune modulating drugs.
Biomarkers of the natural history of type 1 diabetes - Using a combination of large data set analysis, small mechanistic studies, and genetic analysis, we have identified markers that predict the natural history of diabetes and shed light on the mechanisms that underly clinical heterogeneity. Specifically, we are investigating how autoantibody titers correlate with development of disease in first degree relatives and how certain immune cell subsets may predict autoantibody titers. In addition, we are understanding the critical role that altered glucose metabolism may play in the development of auto-reactive T-cells through small clinical studies in humans. And finally, we have used SNP genotyping arrays to identify a variant in a ß-cell gene that correlates with the rapidity of loss of insulin secretion. This work will help to create the next generation of clinical trials that seek to prevent type 1 diabetes.
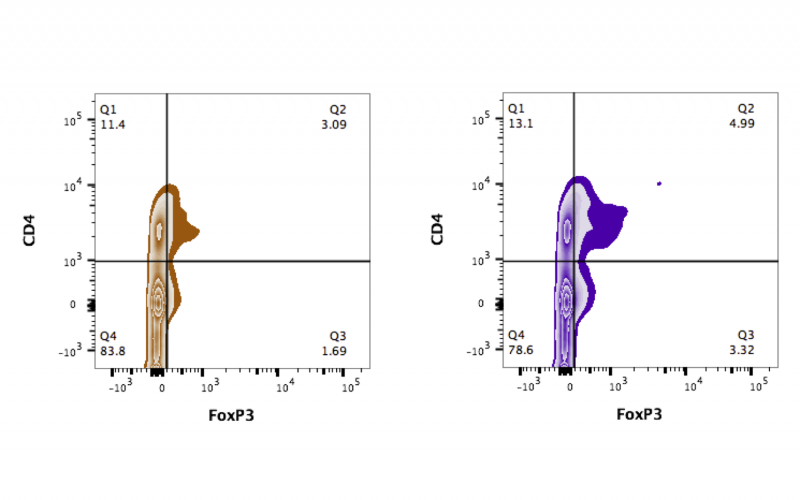
Biomarkers of drug response - We are utilizing human sample analysis to validate a candidate biomarker that correlates with response to immune modulating therapies. We have studied samples from type 1 diabetes patients treated in the rituximab and abatacept clinical trials and have identified a serum based biomarker that correlates with responder status. We are now validating the effect of this candidate biomarker on primary human cells. The above picture demonstrates changes in the transcription factor FoxP3 in primary human T-cells when exposed to the biomarker (orange=no biomarker; purple=cells+biomarker). In the future we will utilize the candidate biomarker as an in vitro screen for identifying new molecules that could be efficacious in preventing type 1 diabetes.
2. Cystic fibrosis related diabetes (CFRD)
We are studying the pathogenesis of CFRD by analyzing human pancreas samples. Our work, in collaboration with Rebecca Hull, PhD at the University of Washington, has shown that the islets of CFRD subjects are characterized by plaques of abnormally folded proteins called amyloid. Amyloid is observed in type 2 diabetes but usually in much older patients. We have also identified a key inflammatory protein, IL-1ß, expressed in the islets of CF patients suggesting that CFRD is associated with pronounced islet inflammation and dysfunction. We are now planning to further our study of the immune process in CFRD by analyzing larger numbers of molecules critical to immune function. Our hope is that by understanding the cause of islet inflammation in CFRD we may be able to target the root cause of islet dysfunction instead of simply treating the effects such as high blood sugars.